In this post, let’s look at fatty acid biosynthesis, oxidation and some important lipids
Lipids are an important group of biomolecules that serve a wide range of functions. They include fats, oils, hormones, and components of the cell membrane.
As mentioned before, lipids are broadly classified as structural and storage lipids based on their functions. Check out my previous post on lipids.
Biosynthesis
Water-soluble precursors like acetate makes up water-insoluble lipids. It is an endergonic and reductive process, meaning, it requires ATP for energy and a reduced electron carrier like NADPH to act as a reductant.
Fatty acids are the primary component of Triacylglycerol and Phospholipids
Biosynthesis of fatty acid
- A very important intermediate of fatty acid biosynthesis is Malonyl CoA, formed by the condensation of Acetyl CoA and Bicarbonate
- Formation of Malonyl CoA is irreversible and carried out by acetyl CoA carboxylase
Malonyl CoA formation
- Let’s look deep into the Malonyl CoA formation
- Carboxyl group of Bicarbonate (HCO3-) is transfers to Biotin (with a butyryl group), which serves as a temporary carrier of CO2
- In the next step, the carboxyl group from biotin transfers to acetyl CoA to form malonyl CoA
- Acetyl CoA carboxylase catalyzes both these reactions
Step | Who is it transferred from | What is transferred | Enzyme | Who is it transferred to |
| 1 | Bicarbonate (HCO3-) | Carboxyl Group | Acetyl CoA carboxylase | Biotin |
| 2 | Biotin | Carboxyl Group | Acetyl CoA carboxylase | Acetyl CoA |
The final product of this reaction: Malonyl CoA, an important intermediate
Charging of fatty acid synthase
The next phase of the biosynthesis of fatty acids is a repeating 4-sequence reaction catalyzed by fatty acid synthase. But, before the 4-sequence reaction proceeds, it is important to charge the fatty acid synthase complex with the right acyl groups. This takes place in 2 steps.
- The Acetyl group of acetyl CoA transfers to ACP with the help of malonyl/acetyl CoA-ACP-transferase (MAT) and the acetyl group from ACP is transfers to the Cys-SH group of β keto acyl-ACP-synthase (KS)
- Malonyl group of Malonyl CoA is transfers to –SH group of ACP with the help of MAT
In short,
Step | Who is it transferred from | What is transferred | Enzyme | Who is it transferred to |
1A | Acetyl CoA | Acetyl group | Malonyl-acetyl CoA-ACP-transferase (MAT) | ACP |
1B | ACP | Acetyl group | Malonyl-acetyl CoA-ACP-transferase (MAT) | Cys-SH group of β-keto acyl-ACP-synthase (KS) |
| 2 | Malonyl CoA | Malonyl group | Malonyl-acetyl CoA-ACP-transferase (MAT) | -SH group of ACP |
4 sequence reaction
Upon charging with the right acyl groups, a 4-sequence reaction proceeds. Acyl Carrier Protein (ACP) is an important enzyme that holds together the entire system. Each cycle extends the fatty acids by 2 carbon units. ACP is a flexible arm attaching the growing fatty acyl chain with the fatty acid synthase. Let’s look at each one of them in detail.
- Condensation: The acetyl group of the Cys-SH group transfers to the malonyl group of -SH of ACP to form acetoacetyl ACP and CO2 (transient). β-ketoacyl ACP synthase catalyzes the reaction
- Reduction: Acetoacetyl ACP has a carboxyl group at its C3 position. This carboxyl group reduces to D-β-hydroxy butyryl-ACP with the help of the enzyme β-ketoacyl-ACP reductase (KR) and NADPH as an electron donor
- Dehydration: removal of water from C2 and C3 of D-β-hydroxybutyryl-ACP. β-hydroxybutyryl-ACP-dehydratase catalyzes the reaction. This introduces a double bond in the product giving trans-Δ 2-butenoylACP
- Reduction of double bond: the double bond reduces to butyric ACP by enoyl ACP reductase and NADPH as an electron donor
So, the 4 reactions in short
Step | Name of the reaction | Substrate | What happens | Enzyme | Product |
| 1 | Condensation | Cys-SH | Transfers Acetyl group to Malonyl group of -SH of ACP | β-ketoacyl ACP synthase | Acetoacetyl ACP+ CO2 |
| 2 | Reduction | Carboxyl group in C3 position of Acetoacetyl ACP | Reduced | β-ketoacyl ACP-ACP reductase (KR) with NADPH as an electron donor | D-β-hydroxybutyryl-ACP |
| 3 | Dehydration | C2 and C3 of D-β-hydroxybutyryl-ACP | Removal of water | β-hydroxybutyryl-ACP-dehydratase | trans-Δ 2-butenoylACP |
| 4 | Reduction | Double bond introduced in step 3 | Reduction of double bond | Enoyl ACP reductase (ER) with NADPH as an electron donor | Butyric ACP |
Upon completion of the four reactions, the butyric group of phosphopantethine transfers to the SH group of ACP. The Malonyl group binds to the free phosphopantethine to start the next cycle.
For reasons still unknown, the 4-step reaction stops after seven cycles giving a 16 C saturated palmitoyl group still bound to ACP. ThioEsterase (TE) removes it from the ACP.
Synthesis of long-chain fatty acids:
Palmitate lengthens to form stearate. Palmitate and stearate act as a precursor of palmitoleate and oleate, both having a double bond in the cis configuration between C9 and C10. fatty acyl-CoA desaturase introduces the double bond.
Palmitate synthesizes other long-chain fatty acids through the same mechanism (4-step condensation and reduction reactions). The only exception is the acyl carrier is Coenzyme A instead of ACP
Lineoleate and alpha-linolenate are essential fatty acids and require supplemenation through our diet.
β oxidation of fatty acid
The breakdown of fatty acids occurs in the mitochondria in three stages. Let’s look at them individually.
Stage 1: β oxidation: it involves the removal of two carbon units from the carboxyl end in the form of acetyl CoA
for eg: palmitic acid, after 7 cycles of β oxidation will yield acetyl CoA
The formation of one acetyl CoA requires the removal of 4 hydrogen atoms by a group of enzymes called dehydrogenase.
Stage 2: acetyl groups (in acetyl CoA) further oxidizes to CO2 in the citric acid cycle. The end of stage 2 produces two reduced electron carriers namely NADH and FADH2 .
Stage 3: NADH and FADH2 produced in stage 2 donate electrons to Oxygen in the respiratory chain and help in the eventual conversion of ADP to ATP
Let’s look at stage 1 in detail. Stage 2 and stage 3 will be addressed as part of the citric said cycle from my previous blog post on carbohydrates and an upcoming blog post on respiratory chain
β oxidation of saturated fatty acids ( single bond in the carbon chain)
β oxidation requires 4 main steps:
- Dehydrogenation of fatty acyl CoA
- This step introduces a double bond between alpha and β carbon to give Trans Δ 2-enoyl-CoA. Acyl CoA dehydrogenase ( it is a flavoprotein FAD complex) catalyzes this reaction. 3 isozymes of this acyl CoA dehydrogenase act according to the length of the fatty acid. The electrons from the fatty acyl CoA transfers to FAD to give a reduced form of dehydrogenase. The reduced form of dehydrogenase transfers its electrons to electron transfer flavoprotein (ETF) of the respiratory chain
- Addition of water to trans-Δ 2-enoyl CoA
- Acyl CoA hydratase catalyzes the reaction to give β hydroxy acyl CoA (3 hydroxyacylCoA)
- Dehydrogenation
- β hydroxy acyl CoA dehydrogenates to β ketoacyl CoA and β hydroxyacyl CoA dehydrogenase catalyzes the reaction. NAD+ accepts electrons to give NADH which donates its electrons to NADH dehydrogenase of ETC eventually passing to Oxygen of ETC which will eventually generate ATP
- Thiolysis
- β ketoacyl CoA + free Coenzyme A= acetyl coA+ Coenzyme A of thioester of a fatty acid (shortened by 2 C). Acyl CoA acetyltransferase also known as thiolase catalyzes this reaction and hence the name.
So, the reactions in short
Step/name of the reaction | Substrate | Enzyme | What it does | Product |
Dehydrogenation | Fatty acylCoA | Acyl CoA dehydrogenase | Introduces a double bond between alpha and β carbon | Trans Δ 2-enoyl CoA |
Hydration | Trans Δ 2-enoyl CoA | Acyl CoA hydratase | Addition of water to Trans Δ 2-enoyl CoA | β hydroxyacyl CoA |
Dehydrogenation | β hydroxyacyl CoA | β hydroxyacyl CoA dehydrogenase | Dehydrogenation NAD+ accepts electrons, give them to NADH which inturn gives it to oxygen of respiratory chain | β ketoacyl CoA |
Thiolysis | β ketoacyl CoA + free CoA | acyl CoA acetyltransferase | Adds Coenzyme A to the substrates | Acetyl CoA + CoA of thioester of fatty acid |
The β ketoacyl CoA along with a free CoA will give acetyl CoA + Coenzyme A of thioester of a fatty acid shortened by 2 carbons.
For fatty acyl chains of 12 or more carbons, trifunctional protein (TFP) catalyzes the last three reactions.
So, one β oxidation removes 1 molecule of acetyl CoA, 2 electron pairs, four protons from a long chain fatty acid shortening it by 2 C
As mentioned previously, acetyl CoA enters the citric acid cycle to convert to CO2 and H2O
Electrons from NADH and FADH2 enter the respiratory chain to eventually give ATP
β oxidation of unsaturated fatty acids (I.e. fatty acids with double bonds)
The reaction is similar to oxidation in saturated fatty acids. However, it requires 2 additional enzymes. This is because the double bonds present in the fatty acid are in cis form and by enoyl CoA hydratase cannot act on them. Let’s understand the 2 enzymes by two separate examples.
Enzyme 1- Isomerase: beta-oxidation of unsaturated fatty acids with a single cis bond (one double bond in a cis configuration)requires these enzymes
Eg: oleate, which has a cis-bond between C9 and C10. Let’s look at the steps
- oleate converts to oleoyl CoA
- oleoyl CoA upon three cycles of beta-oxidation gives 3x acetyl CoA + cis-Δ3-dodecanol CoA
- the product from step 2 has cis double bond and enoyl CoA cannot act on this product. So, Δ3, Δ2-enoyl CoA isomerase converts this product to trans -Δ3-dodecanol CoA
- after the remaining steps of beta-oxidation, it gives acetyl CoA+ decanoyl CoA
- upon 5 more beta oxidation cycles, it gives 5x acetyl CoA
So, 1 molecule of oleate gives a total of 9 acetyl CoA
Enzyme 2- Reductase: beta-oxidation of unsaturated fatty acids with multiple cis bonds (one or more double bonds in a cis configuration) requires these enzymes
Eg: Linoleate has 2 cis double bonds at position C9 and C12
- linoleate, upon conversion to linoleoyl CoA will undergo 3 rounds of beta-oxidation to give 3x acetyl CoA+ CoA ester of a 12-carbon unsaturated fatty acid
- The product of step 1 has double bonds at 2 positions- cisΔ3 and cis Δ6 and enoyl CoA alone cannot act on this product
- Enoyl CoA along with 2,4-dienoyl CoA reductase will facilitate its reentry into the beta-oxidation cycle
- finally gives 6x acetyl CoA
So, 1 molecule of linoleate gives a total of 9 acetyl CoA
Biologically important lipids
Cholesterol
- It forms an essential part of the plasma membrane and also acts as a precursor for the synthesis of steroid hormones and bile acids
- Despite its importance, an excess accumulation of cholesterol in the blood vessels of the heart results in cardiovascular diseases and stroke. This hence requires a balance between its biosynthesis and utilisation
- It is synthesized from acetyl CoA. In this lengthy process, acetate from acetyl CoA converts to isoprene units which condense together to give a linear molecule consisting of 30 carbons. They eventually cyclize to form a four-ringed cholesterol structure
- Cholesterol precursor: Hydroxy Methyl Glutaryl CoA (HMG-CoA), which upon four reactions will give an isoprenoid intermediate called isopentenyl phosphate. It also controls the rate of cholesterol biosynthesis
- What inhibits cholesterol: statins. Most drugs that are cuurently in use for treating high cholesterol have the suffix statin eg atorvastatin. They act by inhibiting HMG-CoA reductase
- Uses:
- precursor of steroid hormones like cortisol, androgens, and estrogens
- The liver converts cholesterol to bile acids- they help emulsify the food we eat and help digest the fats
- Atherosclerosis is a slow and progressive condition resulting from the excessive accumulation of lipids in blood vessels of the heart, especially coronary arteries. The resulting plaque formation consists of a core of cholesterol, cholesterol esters, and dead macrophages surrounded by smooth muscle cells. The plaques end up blocking the vessels forming a blood clot that prevents the blood from circulating to the heart thereby causing a myocardial infarction/ heart attack
Bile salts
- They help in the absorption of triglycerides which we ingest through our food. Upon their conversion from insoluble fat particles to finely dispersed micelles, they absorb into the bloodstream through the intestinal walls. Bile salts carry out the conversion from fat particles to micelles
- As amphipathic compounds, they are biological detergents.
- Through micelle formation, a large amount of fats are exposed to lipase, the enzyme that breaks down fats like triacylglycerols to monoacylglycerols, diacylglycerols, free fatty acids, and glycerol. Once broken down, apolipoproteins help transport triacylglycerols, phospholipids, cholesterols, and cholesterol esters between different organs
Eicosanoids
- They are cell signaling molecules and play an important role in innate immunity
- Different types of eicosanoids like prostaglandins and leukotrienes help protect our immune system by invading bacteria
- They are involved in the production of pain and fever and the regulation of blood pressure
- As paracrine hormones, their effects are primarily local
- A 20-carbon polyunsaturated fatty acid called arachidonic acid is the precursor for eicosanoids
- They are not stored in the cells and are rather synthesized whenever required
- Let’s look at important eicosanoids
- Leukotrienes: They are derived from arachidonic acid by the enzyme lipoxygenase. They have three conjugated double bonds
- Prostaglandins: it is a 20-carbon fatty acid with a 5-carbon ring which upon modification synthesizes 9 classes of prostaglandins (PGA to PGI)
- Thromboxanes: they are derived from a nascent prostaglandin by thromboxane synthase
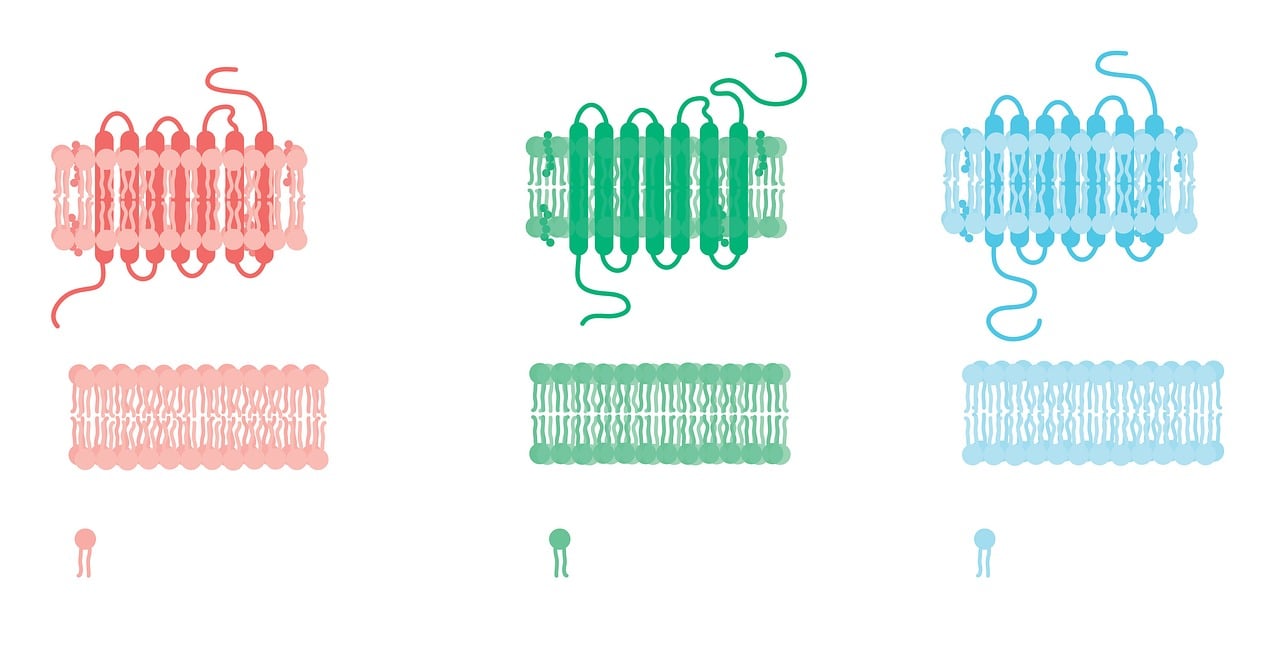
Lipid bilayers
Phospholipids and glycolipids make up majority of the lipid bilayer.
Let’s look at each one in detail.
Phospholipids
Phospholipids, as you have seen previously constitutes four main components:
- Fatty acids
- a platform for attachment of fatty acids
- phosphate, and
- alcohol
Fatty acids, being hydrophobic act as a barrier against the external environment
The platform for fatty acids can be either glycerol (as in phosphoglycerides) or sphingosine (as in sphingomyelin)
The simplest phosphoglyceride is Diacylglycerol-3-phosphate (DAG)
Glycolipids
Glycolipids are sugars containing lipids
Sphingosine is the precursor
The simplest glycolipid is cerebroside with a single sugar residue
Complex ones like gangliosides contain a branched chain of up to seven sugar molecules
Phospholipids are amphipathic (containing hydrophilic and hydrophobic components) and that is why they can form membranes. Polar head groups prefer water and the hydrophobic tails repel water.
Due to this structure, they assemble into micelles and bilayers
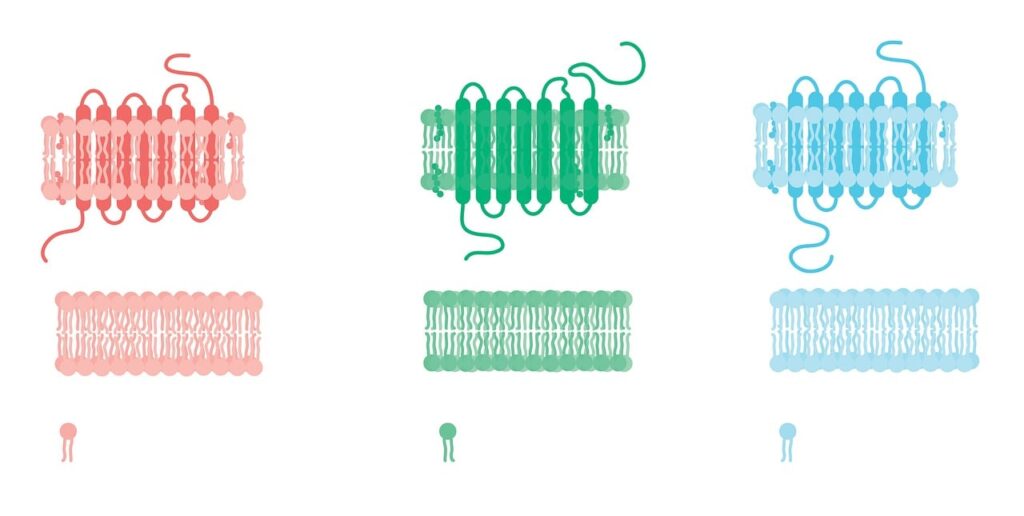

With this arrangement, the hydrophobic tails face the interior and act as a permeability barrier and the polar head group interacts with the aqueous layer
But among the two, nature prefers bilayers as it is difficult to accommodate two fatty acids in a micelle.
Lipid bilayers form spontaneously with the hydrophobic interactions being the driving force. This propensity of phospholipids to form membranes has been exploited in the use of lipid vesicles called liposomes. They are aqueous compartments in a lipid bilayer having therapeutic applications in the form of drug delivery using liposomes. They are often nontoxic and are hence considered safe.
Model membrane systems
Despite the stable nature of the lipid bilayer, its phospholipid molecules and sterol molecules have a certain degree of freedom of motion. Due to this property, the lipid bilayer exists in 3 different phases:
- Semi-solid gel phase– below physiological temperatures which constrains all the motion
- In liquid disordered state– also called fluid state- above physiological temperatures, the interior of the bilayer is more fluid-like
- Liquid ordered state– at physiological temperatures. There is less thermal movement in the acyl chains but there is lateral movement in the plane of the bilayer.
At temperatures falling in the physiological range, long-chain saturated fatty acids pack into a liquid-ordered state. However, the kinks in unsaturated fatty acids interfere with packing and favor the liquid-disordered state
Transport of solutes across the membranes
With the plasma membrane as a barrier between the cell and its external environment, the cell needs to acquire all the necessary raw materials from the extracellular environment. Some non-polar solutes can pass through the membrane unassisted. However polar solutes require the assistance of membrane proteins.
Membrane proteins assist the movement of solute both down and against its concentration gradient. Moving the solute against its concentration gradient requires energy and it may come directly from the hydrolysis of ATP or may be supplied through the movement of one solute down the concentration gradient to release enough energy to drive another solute up its gradient.
Ion channels and ionophores
Ions may move through ion channels (made of proteins) or ionophores (small molecules that mask the charge of ions and allow them to diffuse through the lipid bilayer)
Routes of solute transport
Small molecules are transported across the membrane through one of the three routes: transmembrane channels, carriers, or pumps
Passive transport
When unequal concentrations of a soluble compound are separated by a barrier, the movement of the solute is through simple diffusion. It moves from the region of higher concentration to lower concentration. When ions of opposite charge are separated by a barrier, it generates a transmembrane electrical gradient known as membrane potential Vm. This potential produces an opposing force to the ions that are increasing Vm and producing a driving force for ions that reduce Vm.
Thus, the movement of the direction of ions depends on both chemical and electrical gradients, together referred to as electrochemical gradient and electrochemical potential.
Membrane proteins decrease the activation energy for the transport of polar compounds and ions. Proteins that bring about this passive transport or facilitated diffusion are transporters or permeases.
Transporters fall into two categories: carriers and channels
Carriers bind to their substrate (very stereospecific) and are saturable, more or less like enzymes
Carriers that facilitate diffusion down the concentration gradient are passive transporters and those which facilitate diffusion against their concentration gradient are active transporters.
There are two types of active transport:
Primary active transport is where the accumulation of solute directly couples to an exergonic chemical reaction like the conversion of ATP to ADP. Eg: P-type ATPases, calcium pump, sarcoplasmic and endoplasmic reticulum pumps (SERCA), F-type ATPases, V-type ATPases, ABC transporters
Secondary active transport is where an endergonic/uphill transport of one solute couples with the downhill flow of a different solute. The energy required for secondary active transport is provided by ion gradients
Channels show less stereospecificity and are not saturable.
Ion selective channels act along with pumps such as Na+K+ATPase.
Ion channels are different from ion transporters. They are of two types: ligand-gated channels which bind to an extracellular or intracellular molecule resulting in an allosteric transition. This eventually opens or closes the channel. Voltage-gated channels where a change in transmembrane electric potential causes a charged protein to move relative to the membrane thereby opening or closing a channel.