In this blog post, let’s have a look at proteins and the amino acids that make them up.
Structure
- Proteins are essentially the workhorses of the cell and are present in the form of structural components or enzymes. They play a crucial role in all the essential functions of the body and they are linear polymers consisting of 20 amino acids.
Function
- The functional property of a protein depends on its 3 dimensional structure and the sequence of amino acids dictates how it folds into a 3D structure
- 1 Dimension——- sequence information, 3 Dimension—— biological function
- The different functional groups including alcohols, thiols, thio-ethers, carboxylic acids, carboxamides, and a variety of basic groups attribute to the broad range of protein function
Components
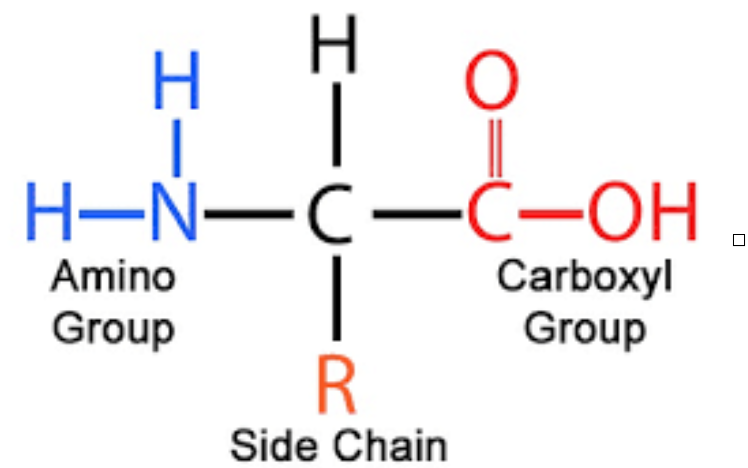
- Amino acids are building blocks of proteins
- They have 5 components:
1. A central carbon atom (alpha carbon)
2. A amino group (NH2)
3. A carboxylic group (COOH) and
4. Hydrogen atom (H)
- Find below the list of amino acids with their type of side chain and single letter codes
| Amino acid | Single letter code | Three letter code | |
Non polar aliphatic R groups | Glycine | G | Gly |
| Alanine | A | Ala | |
| Valine | V | Val | |
| Leucine | L | Leu | |
| Isoleucine | I | Ile | |
| Methionine | M | Met | |
| Cyclic structure | Proline | P | Pro |
Aromatic side chains | Phenylalanine | F | Phe |
| Tyrosine | Y | Tyr | |
| Tryptophan | W | Trp | |
Polar uncharged R groups | Serine | S | Ser |
| Threonine | T | Thr | |
| Aspargine | N | Asn | |
| Glutamine | Q | Gln | |
| Cysteine | C | Cys | |
Negatively charged R group | Aspartate | D | Asp |
| Glutamate | E | Glu | |
Positively charged R group | Lysine | K | Lys |
| Arginine | R | Arg | |
| Histidine | H | His |
- Due to their chirality, there are 2 isomers of amino acids- l and d and they are called enantiomers
- However l amino acids make up all proteins in the biological system
- All amino acids with a chiral carbon (except glycine which is achiral) are optically active as they rotate plane polarised light
Properties - Amino acids exist as Zwitterions as they possess both positive and negative charge
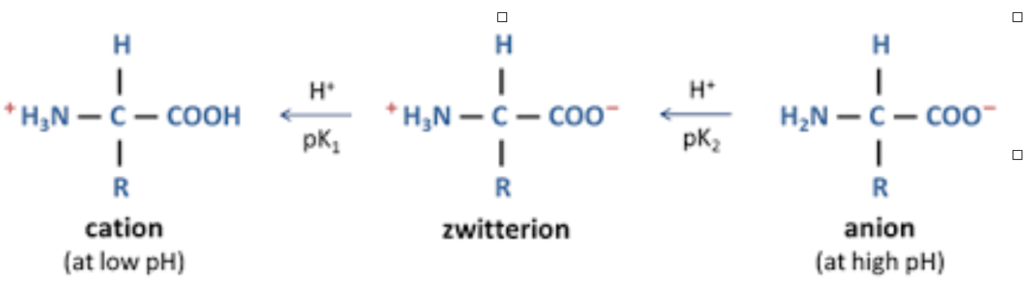
An amino acid as a zwitterion. It exists as a cation in acidic pH and anion in basic pH
- At low pH, they accept a proton, making them cationic with a net positive charge
- At high pH, they donate a proton, making them anionic with a net negative charge
Amino acids and why their R groups are special
Amino acid | R group | Key Feature |
Alanine, valine, leucine, isoleucine | Non polar aliphatic R groups | Cluster together within the proteins |
Glycine | Non polar aliphatic R groups | Simplest and smallest side chain, found in loops and turns |
| Methionine | Sulphur containing R group | Has a non polar thioether group |
| Proline | Non polar aliphatic R groups | Cyclic structure, has an imino group |
Peptide bond– the bond that stabilises the protein structure
- The linking of carboxyl group (COOH) of one amino acid to the amino group (NH2) of the next amino acid through a peptide / amide bond forms the primary structure of proteins and the formation of this bond accompanies loss of water
- A typical protein contains 50 to 200 amino acids
- The molecular weight of an amino acid residue= 110 Da
- Approximate molecular weight of a protein is between 5500 to 220000 Da
Structure of peptide bond
- The peptide bond is essentially planar and has a partial double bond character, thereby restricting its rotation around this bond. The double bond character is due to partial sharing of two pairs of electrons by the carboxyl and amide group. Despite its partial double bond character, the bond between amino group and carbon (phi bond) and carbon and carboxylic group (psi bond) is purely single bond, hence allowing effective folding (freedom of rotation) to give a 3 dimensional protein structure
- The C-N distance in the peptide bond is 1.32 angstrom
- The peptide bond takes up 2 forms- cis and trans. But, most proteins are in trans form. The preference to trans form over cis is due to stearic hindrance. Only X-Proline linkages are in cis form
Secondary structures of proteins
The polypeptide chain can fold into different structures. Let’s look at each one of them in detail
- Alpha helix
Tightly coiled structure stabilised by hydrogen bonds (CO-NH bonds).
The CO bond of one residue forms a hydrogen bond with the NH bond of another residue located 4 amino acids away.
Distance between an amino acid residue- 1.5 angstroms
Rotation- 100 degrees
3.6 residues per turn
They may be right handed or left handed but right handed helix is more energetically favourable as it avoids stearic hindrance
Where are the found? Myosin, tropomyosin, keratin - Beta pleated sheet
Fully extended structure linked through hydrogen bonds
Distance between amino acids- 3.5 angstrom
They may be parallel or antiparallel
Where are they found? Fatty acid binding proteins
Rigid and well defined structures
They participate in interaction between proteins and other molecules
Linking between CO of residue I to NH of residue I+3
Tertiary structure of proteins
- This is the 3 dimensional structure of a protein. The biological function of the protein depends on this structure
- Let us understand this using an example- Myoglobin
- Myoglobin is the oxygen carrier of the muscle. It is an extremely compact single polypeptide made of 153 amino acids
- Heame, a non protein helper group ( a proto- porphyrin ring and an iron molecule in the centre) in myoglobin helps in oxygen binding
- The interior of myoglobin contains non polar amino acids like valine, leucine, methionine, which indicates that hydrophobic residues are present away from the aqueous environment. The only charged amino acid in the interior of myoglobin is histidine, which plays a crucial physiological role
Quaternary structure of proteins
- The quaternary structure of protein arise when a protein consists of multiple polypeptides
- A classic example of such a structure is haemoglobin, consisting of two alpha and two beta subunits. The four subunits collaborate together to perform its function of carrying oxygen from lungs to the tissues
Classification of proteins based on shape
Considering the higher levels of structure, proteins fall into two categories: fibrous and globular based on their shape
Fibrous protein
- Polypeptide chains arranged in long chains or strands
- Usually consists of a single type of secondary structure and they have a relatively simple tertiary structure
- Role- they provide shape, support and external protection
- Examples- alpha keratin, collagen
Globular protein
- Polypeptide chains arranged in spherical or globular shape
- They contain several types of secondary structures
- Role- enzymes and regulatory proteins
Denaturation and renaturation of proteins
- Denaturation is a loss of 3 dimensional structure which is sufficient to cause a loss of function. Christian Anifsen, in the 1950’s, worked on the denaturation of proteins to study the relationship between the tertiary and primary structures of a protein.
- The most common method of protein denaturation is heat, which destroys the hydrogen bonds that primarily make up a protein
- Urea and guanidium chloride cleaves the non covalent bonds in the proteins could be cleaved and Beta mercaptoethanol (a reducing agent) cleaves disulphide bonds. This way, disulphides like cystitis are converted to sulfhydryls like cysteines. Similarly, there are various other reagents used for denaturation of proteins. Some reagents have specificities towards certain amino acids eg. trypsin has specificity for lysine and arginine
- The information required to specify the catalytically active structure of ribonuclease is contained in its amino acid sequence
- In addition to chemicals, proteins can also be denatured by heat
- Each amino acid have a propensity to fold into either alpha helices or beta sheets
- While valine and isoleucine have a propensity towards beta sheets, alanine, glutamate and leucine have a propensity to form alpha helices. Glycine, asparagine and proline tend to be found in loops
- Why do specific amino acids have these preferences? Valine, threonine and isoleucine tend to destabilise an alpha helix due to stearic clashes and they are readily accommodated in the beta sheets as their side chains project out of the plane
Purification of proteins
- The first step in a protein purification method is to break open its source. The source of a protein can either be a tissue or a microbial cell and breaking them will release the proteins in a solution called the crude extract. If the protein is in a specific organelle, an addition differential centrifugation is done in order to first separate the organelle and then release the protein into the crude extract
- The next step is fractionation, to separate the protein based on size or charge. Initial fractionation steps utilise solubility, which depends on pH, temperature, salt concentration and other factors
- High salt concentration—— low solublity- salting out. Certain salts when added at the right amount can precipitate out proteins. Eg. ammonium sulphate is generally used for this purpose
- The next step is to separate the proteins from the remaining solution through low speed centrifugation
- Some proteins require subsequent purification steps like dialysis, which separates the protein from its solute on the basis of the size of the protein
- In addition to these purification techniques, there is a more powerful technique for fractionating proteins I.e. column chromatography which takes advantage of the differences in protein charge, size and binding affinity
- Other types of purification techniques include affinity chromatography (based on the chemical composition and its affinity to the ligand), ion exchange chromatography (to isolate proteins based on charge) or even modern versions of chromatography like high performance liquid chromatography
- Another classic technique used for separation and isolation of a protein is SDS PAGE (Sodium Dodecyl Sulphate Poly Acrylamide Gel Electrophoresis). This technique is used for isolating a single protein from a mixture of proteins. It separates the proteins on the basis of size. Two Proteins of the same size can be separates using a 2 D SDS PAGE

2 thoughts on “Amino acids and proteins”
Comments are closed.