Enzymes are proteins that act as biological catalysts. All enzymes are proteins, but, not all proteins are enzymes. As catalysts, they mediate different biologically important reactions. All through my previous posts, you have seen several reactions catalysed by enzymes. This shows their importance in biochemistry.
Nomenclature
Enzymes end with the suffix -ase. Let’s look at some examples
Urease– catalyses the hydrolysis of urea
Alcohol dehydrogenase– catalyses the oxidation of alcohols to aldehydes and ketones
While the function or substrate of these enzymes were clear, for some enzymes like catalase, it was unclear, due to which a nomenclature for enzymes was adopted by the International Union of Biochemistry and Molecular Biology (IUBMB).
According to the nomenclature, each enzyme gets two names: an accepted name (for everyday use) and a systematic name (name of the substrate followed by -ase). It also gets a four digit classification number.
Classification
There are six major classes of enzymes. They are:
- Oxidoreducatses- redox reaction
- Transferases- transfer of groups
- Hydrolases- hydrolysing a bond
- Lyases- to form double bonds
- Isomerases- isomerization
- Ligases- formation of a bond
Cofactors
Sometimes, enzymes need help with the oxidation-reduction reactions or group transfer reactions. That is when cofactors, known as enzyme’s chemical teeth come to the rescue! They could be metal ions like Cu2+, Fe3+ etc.
Coenzymes
When a cofactor is an organic molecule, it is called coenzyme.
Co-substrates
Some coenzymes are only partly associated with the enzymes and are hence called co-substrates. Nicotinamide Adenine Dinucleotide (NAD+) and Nicotinamide Adneine Dinucleotide Phosphate (NADP+) are well known examples.
Prosthetic groups
Some cofactors are permanently attached to the proteins by covalent bonds. For eg cytochromes with a heme prosthetic group.
Holoenzymes and Apoenzymes
An enzyme-cofactor complex which is catalytically active is a holoenzyme.
If we remove the cofactor, the resulting inactive protein is an apoenzyme.
Inactive apoenzyme + cofactor= active holoenzyme
Activation energy
How do enzymes catalyse reactions? Our understanding of this came primarily through the transition state theory developed by Henry Eyring.
Let’s understand the theory using an example:
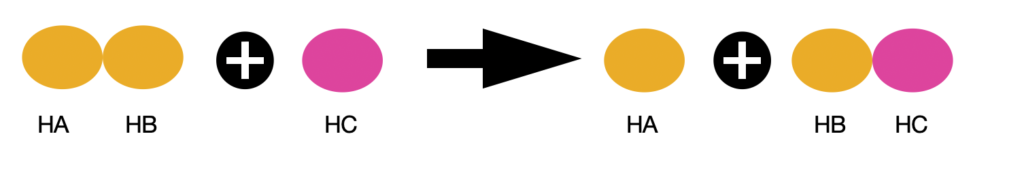
For this reaction to happen, it most certainly will have a highly unstable intermediate

At this point, the complex has the highest free energy known as the transition state. The energy difference between the ground state and transition state is called activation energy / free energy.
If the energy of transition state is less than that of the reactants, it is called free energy of activation (∆G). Greater the value of ∆G, slower is the reaction rate. Catalysts (enzymes) act by reducing the free energy of a biological reaction and hence increases the rate of the reaction. Therefore, the reaction proceeds faster.
However, it must be noted that enzymes only increase the rate of the reaction but do not affect the reaction equilibrium.
Active sites
All enzyme catalysed reactions take place in a specific region on the enzyme called the active site. During the reaction, the substrate binds to the active site of the enzyme.
Let’s look at some common features of active sites.
- Its 3 dimensional structure consists of several groups coming from different parts of the amino acid sequence. Residues which are far apart in an amino acid sequence can interact more strongly than residues close to each other
- It is a tiny dot (takes up very little volume) on the enzyme. To get an understanding of the size of the enzyme when compared to its active site, let’s look at an example. Most enzymes consists of nearly 100 or more amino acid residues and hence their mass is greater than 10kd and have a diameter of almost 25 Angs. The extra amino acids is what forms the active site by acting as a scaffold.
- They are often clefts/ crevices: the substrate molecules bind to these clefts. Water molecules are generally excluded from the clefts unless they are reactants. What makes the substrate-active site bond strong is its non covalent nature. Despite the active site being completely non polar, they also contain certain polar residues. They attain special properties essential for substrate binding.
- The enzyme-substrate interactions is through multiple weak interactions. They are bound by non covalent bonds like hydrogen bonds, electrostatic interactions, van der weals forces and hydrophobic interactions which are much weaker than covalent bonds. They mediate a reversible binding of substrate to the enzyme, which means the enzyme and the substrate should be complimentary to each other.
- How specifically they bind depends on precisely defined arrangements of atoms in the active site.
Theories of enzyme- substrate interactions
Two different theories explain the complimentary interaction between enzymes and substrates.
- Lock and Key model
Here, the active site of the unbound enzyme is exactly complimentary in shape to the substrate - Induced-fit theory
Here, the enzyme changes shape after it binds to the substrate. Precisely, the active site of the enzyme forms a shape that is complimentary to the substrate only after they bind together.
Enzyme specificity: how are enzymes so specific?
It is due to two things:
- Substrate
- The way their catalytic groups are arranged
Mechanisms of enzyme action
Enzymes act through five different types of catalytic mechanisms. Let’s look at it in detail:
- Acid-base catalysis:
In this case, the free energy of the transition state is reduced due to the transfer of a proton from an acid. This is called acid catalysis
Eg: tautomerization of keto to enol is very slow when uncatalyzed due to it’s carbanion transition state. However, upon the donation of a proton to the oxygen atom, the carbanion character of the transition state is reduced making the reaction proceed faster.
Note that this mechanism can also work if a proton is removed from a base known as base catalysis. Some reactions employ both these catalysis simultaneously and is hence called the acid-base catalysis. This mechanism is pH sensitive.
Amino acid side chains like Asp, Glu, His, Cys, Tyr and Lys are acid/base catalysts. - Covalent catalysis:
It increases the speed of a reaction by forming a covalent bond (temporary) between the substrate and the catalyst.
a nucleophilic group of the catalyst reacts with the electrophilic group of the substrate results in the formation of covalent bond between the two. This mechanism is hence also called nucleophilic catalysis.
A classic example of this mechanism is seen in the decarboxylation of aceto- acetate. It is catalysed by primary amines. The nucleophilic amine attacks the carbonyl group of acetoacetate to give a schiff’s base (imine) with a protonated nitrogen. This acts as an electron sink and thereby reduces the high energy of the transition state.
Covalent catalysis essentially occurs in three stages:
Catalyst + substrate= Catalyst —–Substrate (electrophilic catalyst)
Removal of electrons by the electrophilic catalyst
Removal of the catalyst
A good catalytic group must be able to form a strong covalent bond as well as decompose at the end of the reaction. Groups like imidazole and thiols groups are good covalent catalysts. Thiamine pyrophosphate and pyridoxal pyrophosphate (which you will be looking at shortly) are good examples of covalent catalysts. - Metal ion catalysis
Many enzymes require metal ions like Fe3+, Cu2+, Mn2+ etc as a cofactor. These metals are called metalloenzymes. These cofactors are mostly transition metals. They help enzymes in three ways:
By binding to the substrate and adjust their orientation
By carrying out redox reactions
By shielding the negative charges
Lets look at a well known reaction catalysed by carbonic anhydrase to understand this mechanism
CO2 + H2O= HCO3- + H+
The enzyme has a Zn2+ ion surrounded by three His side chains in its active site. The Zn2+ polarises a water molecule to form an OH- group which attacks the CO2 to give HCO3- - Proximity and orientation effects
For a successful reaction to occur, the two reactants (or more) must come together in a proper spacial arrangement.
Eg: Imidazole + p-Nitrophenylacetate= N-Acetylimidazolium + p-Nitrophenolate
the same reaction intramolecularly is 24X faster! When imidazole is covalently attached to the reactants, it is more effective.
Just by bringing the two reactant in close proximity, a reaction proceeds faster. How does this happen?
It could happen in several ways:
Catalytic groups of both the reactants come close to each other
The enzyme when bound to the substrate in a specific orientation, the reaction is faster
Charged groups of the reactants can help stabilise the transition state of the reaction through a process of electrostatic catalysis
The enzymes freeze the translational and rotational motions of the substrates and catalytic groups. This is one of the most important mechanism of increasing the rate of the reaction - By binding to transition states
We have seen cases of enzymes binding to the substrates. However, a yet another crucial mechanism involves the binding of enzymes to the transition state. In fact, they bind to them stronger than they bind to substrates.
Their importance extrapolates to the clinical scale as well. Because the enzyme-transition state interaction is very important, analogues of transition states are often used as enzyme inhibitors.
Enzyme activity and specific activity
The above two terms are often used when it comes to enzymes. Let’s look at their differences.
Enzyme activity is the number of substrates transformed to a product every unit time. I.e. How much of the substrate is being converted to products in a given amount of time?
Although this quantifies the live number of enzyme molecules in a particular process. A variety of factors like temperature, acid levels, substrate concentration etc affect this assessment.
The SI unit of enzyme activity is Katal. However, citing practical difficulties, enzyme activity is the Enzyme Unit (U) which is µmol per minute.
Specific activity is the activity of the enzyme per milligram of protein i.e. what is the enzyme activity (rate of substrate to product conversion) in 1 mg of total proteins. It hence dictates how pure an enzyme is within a given protein. Its significance can be seen in the effective separation of enzymes from specific proteins in order to measure enzyme purity.
The SI unit of specific activity is katal per kg. It is often expressed as µmole per mg per minute, which is in fact the enzyme’s rotation number.
Let’s look at some important coenzymes & cofactors and their role
NAD+
Nicotinamide Adenine Dinucleotide is an important coenzyme for redox reactions. It hence plays a central role in energy metabolism. Check out my previous blog post to understand the function of NAD in aerobic respiration. It helps transport high energy electrons to the electron transport chain during an aerobic respiration.
In addition to their role as a coenzyme, they are also an important cofactor for many non redox enzymes like the sirtuins and Poly (ADP-ribose) polymerases (PARPs).
NADP
Just like its relative NAD+, Nicotine Amide Dinucleotide Phosphate (NADP) also acts as a coenzyme in electron transfer reactions. It is the final acceptor of electrons in plants. It is hence an important enzyme in photosynthesis. Once it accepts an electron, it becomes NADPH (it’s reduced form). NADP+ is also a substrate and acts on the enzyme dihydrofolate reductase.
FAD/FMN
Flavin adenine dinucleotide is very similar to NAD+. It contains riboflavin (derived from vitamin B2), adenine, ribose, and phosphate groups. They exist as -Di or -Mono nucleotides (FMN). One can find FMN in the first complex of the ETC.
NADH+ + H+ +FMN———FMNH2 + NAD+
It eats as a coenzyme and cofactor for many enzymes including alpha-ketoglutarate, succinate dehydrogenase etc.
It exists in three states:
- Quinone- FAD- fully oxidised- yellow
- Semiquinone- FADH- partly reduced- Blue
3. Hydroquinone- FADH2- fully reduced- colourless
FAD structure
It contains an adenine nucleotide and a flavin mono nucleotide (FMN) bridged by phosphate group
Functions
- Catalyse redox reactions
- FAD- strong oxidising agent
- Part of metabolic pathways
- ETC and ATP synthesis
- DNA repair
- Nucleotide biosynthesis
- Beta oxidation of fatty acids
- Amino acid classification
- Synthesis of other cofactors
Coenzyme A
Also known as CoA, CoASH, and HSCoA
It is involved in the synthesis and oxidation of fatty acids and oxidation of pyruvate in citric acid cycle. It works closely with the acyl carrier protein.
Structure:
It is a complex in itself consisting of ADP+ 4-phosphopatothenic acid+ beta alanine+ betamercaptoethylamine
Functions
Energy production: crucial component during CO2 and ATP synthesis
Fatty acid synthesis: acts as a helper molecule facilitating oxidation pathway. It carries fatty acids from mitochondria to cytosol.
Boosts drug and enzyme functioning: also used in certain medications to prolong the half life of the medicine.
Thiamine pyrophosphate (vitamin B1)
- It is an important cofactor for enzymes involved in decarboxylation like pyruvate dehydrogenase and alpha-ketoglutartate dehydrogenase (both involved in glycolysis)
- It is involved in oxidative phosphorylation and transketolase reactions
- It plays a crucial role in carbohydrate and amino acid metabolism
- It is a coenzyme for transketolase which cleaves ribose-5-phosphate to D-glyceraldehyde 3 phosphate. TPP decarboxylates hydroxypyruvate in presence of an aldehyde acceptor
Pyridoxal phosphate (vitamin B6)
- It is a cofactor of glutamic acid decarboxylase (GAD). It helps convert glutamate to GABA
- It is a coenzyme in most transamination reactions- decarboxylation, deamination and racemization
- It is involved in the formation of neurotransmitters like serotonin and dopamine
- It is converted to pyridoxine phosphate (pyridoxine) during the transamination of amino acids
Lipoic acid
- It is an antioxidant made by the body, which helps convert glucose to energy
- It is used as a medication to manage and treat diseases involved in oxidative stress like diabetic neuropathy
- Other health benefits include reduced skin ageing, shows memory loss, promotes healthy nerve function, reduce inflammation and lowers the risk of heart diseases
- In addition to being an antioxidant, it is an essential cofactor for oxidative de carboxylation of alpha keto acids
Biotin (vitamin B12)
- It is a coenzyme for five carboxylases: pyruvate carboxylase, 3-methyl carboxylase, propionyl CoA carboxylase, and acetyl CoA carboxylase 1 and 2
- It is a major cofactor involved in CO2 metabolism. They are also used to metabolise carbohydrates, fatty acids and amino acids
- As a cofactor, it aids in the transfer of CO2 groups to target macromolecules
- It is involved in the synthesis of isoleucine and valine
- It is necessary in gluconeogenesis, lipogenesis, and elongation of essential fatty acids
Tetrahydrofolate
- It is a derivative of folic acid (vitamin B9)and is a coenzyme involved in amino acid and nucleic acid synthesis. Also known as one-carbon metabolism, it is involved in single carbon transfer reactions
- Folic acid is converted to its coenzyme form with the help of the enzyme dihydrofolate reductase
- Methotrexate acts on dihydrofolate reductase and hence acts as an inhibitor of folic acid
Metal Ions
- Metal ions play a crucial role in our body by acting as cofactors
- They play a catalytic role in several electron transfer reactions
- Examples include magnesium, Manganese, Zinc, Molybdenum, Cobalt and Copper
- They primarily serve as electron donors or acceptors, Lewis acids or Structural regulators
- They play an important role in the stability and activity of the enzyme
- Not all metal ions act as coenzymes. Some metals like cadmium and mercury tightly bind to certain amino acid residues on the enzyme and make it inactive
Enzymes from thermophilic bacteria
- The importance of retrieving enzymes from thermophilic bacteria (bacteria that can thrive in extreme temperature) is due to their stability at high temperatures
- Examples of there stable enzymes include proteases, amylases, lipase, xylanases etc
- Thermophiles include Bacillus stearothermophilus, Thermus aquatics etc. they thrive under very high temperatures like 45 to 80
- These enzymes can be used to catalyse reactions under harsh environmental conditions
- Advantages of such enzymes include higher substrate solubility, easy mixing and a low risk of contamination
- Applications of such enzymes include the chemical, food, pharma and textile industries
