- Lipids, an important and diverse group of biological compounds are charecterised by their insolubility in water. Being a structurally diverse group of compounds, they also perform a diverse range of functions
- Based on their functions, they are broadly classified as storage lipids and structural lipids. Let’s look at each one of them in detail
- Storage lipids: Lipids are usually stored as either fats or oils, both of them derived from hydrocarbon derivatives called fatty acids.
Let’s have a look at fatty acids, their structure and nomenclature
- Triacylglycerols, made of 3 or more fatty acid residues are named according to their placement on the glycerol moiety
- Fatty acids always end with an -ate and it becomes an -oyl in a fatty acid ester
- For an unbranched fatty acid, the nomenclature includes the chain length and number of double bonds separated by a colon. For eg:
- 18:1(9) cis-9-Octadecenoic acid
- 20:5(5,8,11,14,17) Eicosapentaenoic acid (EPA)
The first number i.e 18 and 20 in this case denotes the chain length, 1and 5 denotes the number of double bonds and the numbers in bracket includes the position of double bonds
Fatty acids
- Fatty acids are carboxylic acids with a hydrocarbon chain. The length of the chain can range from 4-36 carbons. The chain is either branched or unbranched and they are saturated (no double bonds) or unsaturated. In addition, some fatty acids may also have three carbon rings, hydroxyl groups or methyl group branches
- The most predominant fatty acid residues are those with C16 and C18 species and they include: palmitic, oleic, linoleum and stearic acids
Physical property
- The physical property of a fatty acid largely depends on the length of the hydrocarbon chain and degree of unsaturation. The above factors mainly affect the solubility of the fatty acid. The longer the fatty acyl chain and fewer the double bonds, the lower is its solubility in water. The polar carboxylic acid contributes to the slight solubility of short chain fatty acids
- These factors also affect the melting point of the fatty acids. For instance, at room temperature, saturated fatty acids which have 12 – 24 carbon atoms have a waxy consistency and unsaturated fatty acids of the same lengths are liquid at room temperature. The free rotation around the C-C bond gives the fully saturated fatty acids it’s flexiblity
Stable conformation
- The most stable conformation of a fully saturated fatty acid is a fully extended form which minimises the stearic hindrance of neighbouring atoms. They can together pack together to form crystal like arrays. In unsaturated fatty acid, the formation of double bond introduces a kink in the hydrocarbon chain. Due to this, they cannot pack together as tightly as fully saturated fatty acids. Due to the less thermal energy required to disorder these poorly arranged fatty acids, they have lower meting points
Essential fatty acids
- In the blood of vertebrate animals, free fatty acids circulate through a covalent connection with a protein carrier, albumin. However, most fatty acids exist as derivatives of carboxylic acids like esters or amides
- Humans cannot synthesise essential fatty acids and are yet necessary in the body. Dietary supplementation should ensure the availability of polyunsaturated fatty acids (PUFA).Two families of essential fatty acids I.e omega 3 (alpha- linoleum acid) and omega 6 (linoleic acid) are important. A healthy formation of plasma membrane requires essential fatty acids
Triacylglycerols: fatty acid esters of glycerol
- They are the simplest lipids, composed of 3 fatty acids . Each of the three fatty acids links to a single glycerol through an ester linkage
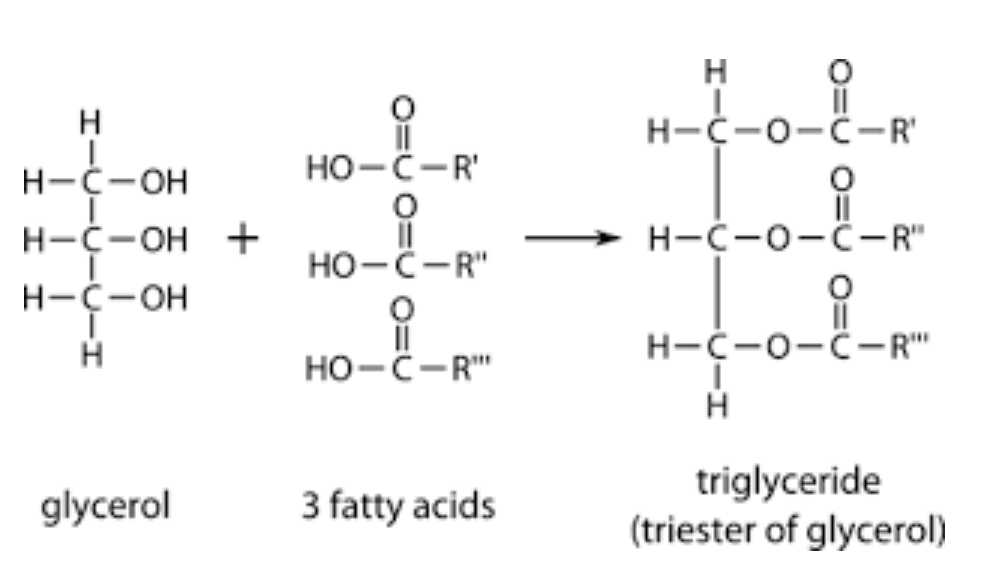
Components of a triglyceride
- Triacylglycerides containing the same type of fatty acids are simple triacylglycerides eg. tripalmitin, tristearin and triolein. However, most naturally occurring triglycerides contain different types of fatty acids
What makes triacylglycerides insoluble in water?
- The polar hydroxyl groups of glycerol and the polar carboxylates of fatty acids are bound in ester linkages. This is why they are non polar, hydrophobic and insoluble in water
Why is a mixture of oil and water immiscible?
- It is due to the lower specific gravities than water. That is why oil floats on water
Triacylglycerides and insulation
- Triacylglycerides are storage lipids and they are also involved in insulation. Inside the cytoplasm, they occur as tiny droplets, serving as depots of metabolic fuel
- Storage of triacylglycerides are in specialised cells called adipocytes in vertebrates. They are also stored in many types of seeds in plants, providing energy and precursors during seed germination. Adipocytes and germinating seeds contain lipase, which catalyze the hydrolysis of stored triacylglycerols. This way, they release fatty acids to sites where they are necessary
- Their advantage over other forms of stored fuels like glycogen and starch?
- Carbon atoms of fatty acids are more reduced and when oxidised, they give two times more energy than carbohydrates
- Because fatty acids are hydrophobic, they do not carry the extra weight of water of hydration, as opposed to story carbohydrates
- Triacylglycerides and insulation: they usually serve this function in seals, walruses, penguins and other warm blooded polar animals. In hibernating animals, they serve a dual purpose of insulation and energy storage. The basis of the remarkable function of triacylglycerides is its low density. In sperm whales, a combination of triacylglycerides and waxes allows the animal to match the buoyancy of their bodies to that of their surroundings during deep dives in cold water
Why do chips turn rancid?
- Upon exposure of chips (rich in unsaturated fatty acids) to oxygen for too long, they become rancid giving an unpleasant taste and smell. This results from the oxidative cleavage of their double bonds. The cleavage results in the production of highly volatile short chain aldehydes and ketones
- Waxes: they are a yet another storage form of lipids
- They are esters of long chain saturated and unsaturated fatty acids with long chain alcohols
- They have a higher melting point than triacylglycerols
- They have important roles in the protection of hair and skin keeping it lubricated and water proof. Birds keep their feathers water repellant by secreting waxes from their preen glands
Structural lipids:
- Lipids form an important part in the architecture of plasma membranes. The plasma membrane consisting of a bilayer of lipids, a sting as a barrier between the cell and the extracellular space.
- Lipids in the plasma membrane are amphipathic I.e. one end of the molecule is hydrophobic and the other end is hydrophilic
- Lets have a look at important lipids
Phospholipids/ glycerophospholipids
- Structure: two fatty acids attaches to the first and second carbons of glycerol through ester linkage. And to the third carbon, a highly polar charged group attaches through a phosphodiester bond
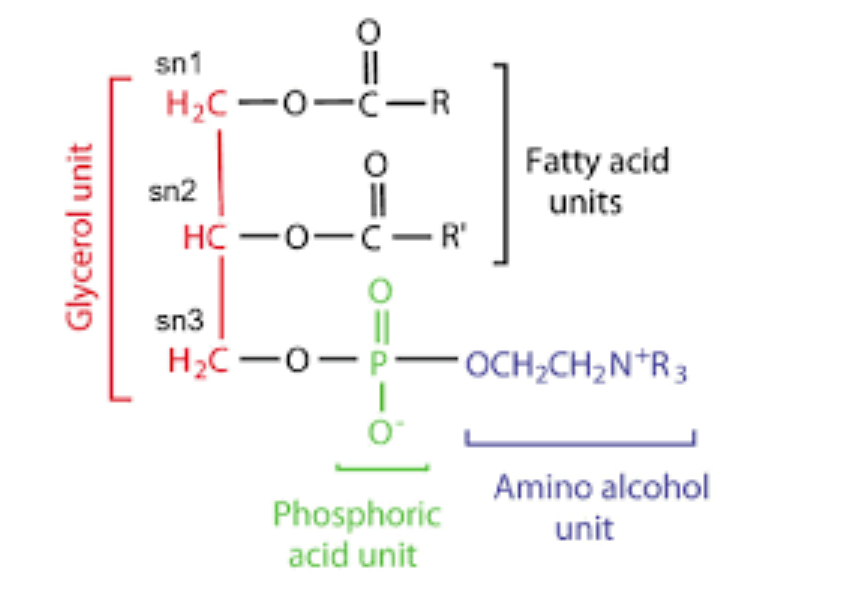
- Phospholipids arise from phosphatidic acid and are components of the plasma membrane
- Phosphate has a net negative charge at neutral pH. The alcohol group which is polar may be negatively charged (phosphotidyl inositol 4,5-bisphosphate), neutral (phosphatidyl serine) or positively charged (phosphotidyl choline and phosphatidyl ethanolamine)
- Plasmalogens contain an ether linkage instead of an ester linkage
- Some lipids such as glycolipids may have a simple sugar or complex oligosaccharide in their polar ends
Sphingolipids:
- Most sphingolipids are components of the amino alcohol sphingosine with a trans double bond. Ceramides are N acetyl fatty acid derivatives i.e. fatty acid attached to a NH2 through an amide linkage. They in turn act as a parent compound for other sphingolipids such as sphingomyelins, cerebrosides, gangliosides
- Sphingomyelins: they are ceramides bearing either a phosphocholine or a phosphoethanolamine head group. They are an important component of the myelin sheath in neurons that electrically insulates the neuron
- Cerebrosides: they are ceramides with head groups consisting of a single sugar residue. They are therefore glycosphingolipids. Most prevalent ones are galactocerebrosides and glucocerebrosides. Because they lack a phosphate group, they are non ionic. They are usually found in the plasma membranes of cells in neural tissue and in some non neural tissues containing glucose in them
- Gangliosides: they are highly complex. They have an oligosaccharide attached to them. This includes at least one silica acid reside they are mostly found in the brain. They have a characteristic N-acetylneuraminic acid (Neu5Ac), a sialic acid at its terminus. This is what gives it a negative charge
Cholesterol
- Steroids are structural lipids found in the cell membrane. They have a characteristic steroid nucleus made of four fused rings. Among the four rings, three of them have 6 carbons and one has 5
- Cholesterol, the major steroid in animals classifies as sterol due to an -OH group on C3. It is amphipathic with a polar head group and a non polar hydrocarbon body
- Similar sterols: plants- stigmasterol, fungi- ergosterol
- A simple five carbon isoprene subunit synthesises sterols of all eukaryotes
- They make up 30-40% of the plasma membrane. The fused ring of cholesterol gives its rigidity
- Bile acids, derived from cholesterol act as detergents of intestine by emulsifying the dietary fats to make them available for lipase
Prostaglandins
- They are an important group of lipids which act like hormones and play an important role in dealing with injury and illness. They are different from hormones as they are not released from a specific gland
- They form at sites of tissue damage or infection and control processes such as inflammation, blood flow, formation of blood clots and even induction of labour
- They basically play a key role in generating inflammatory responses and are synthesised in high levels in inflamed tissues thereby contribute to signs of acute inflammation
- They are present in significantly high concentrations during menstruation. The breakdown of endometrial lining of uterus releases a large amount of prostaglandins. They constrict blood vessels in the uterus and contract the muscle layer causing painful cramps
- They are synthesised from arachidonic acid and are often autocrine and paracrine mediators acting on platelets, endothelium, uterine and mast cells
- What makes this group of lipids more interesting is the fact that they can have different effects based on which receptors they bind to

1 thought on “Lipids”
Comments are closed.